Introduction
The Indian pharmaceutical market is the 3rd largest in the world by volume and 13th largest in terms of value. It has an estimated value of 41 Billion USD1. India is the largest provider of generic drugs, one of the largest suppliers of low-cost vaccines and has provided access to affordable HIV treatment. India accounts for 60% of global vaccine production and is often referred to as the "Pharmacy of the world".2
The Indian pharmaceutical industry has segments dealing with API (Active Pharmaceutical Ingredient) which is the core chemical molecule in pharmaceutical products; OTC (Over The Counter) drugs which can be bought without a doctor's prescription; Generic Drugs which are created to be the same as an already marketed brand-name after the patent of the original drug has expired; Vaccines for several diseases; Contract Research and Manufacturing; and Biologics and Biosimilars.
While traditionally, the pharmaceutical retail operations have been highly localised, advent of technology has led to disruptive innovations in last mile retail offerings to consumers.
E-pharmacies are quite widespread in the country, particularly cities, and appear to be profitable. Lower prices, discounts, convenience in placing orders and home deliveries of medicines has become a game changer for many and the pains of looking and going to a brick and mortar retailer has been minimized.
India, with an already successful and growing pharmaceutical industry, is bound to make the e-pharmacy market grow. Factors such as rapid interest penetration, Digital India Initiative and e-healthcare initiatives by the Indian government, changing disease patterns, growing number of lifestyle diseases, increasing aging population, increased spending on medicines and a booming economy, make ventures in this sector extremely lucrative and attractive.
Being a new type of business, which deals with prescription drugs being highly controlled, it is expected that e-pharmacies shall be at the receiving end of much scrutiny and detailed regulations may be brought into force.
In the absence of much information or regulations in this field and the Indian ecosystem still grappling with the modalities of dealing with e-pharmacies, this blog post seeks to throw light on the system as it prevails and what to expect.
What are E-Pharmacies?
With the advent and rapid growth of the internet, coupled with the booming e-commerce market in the last decade, the advantages of these technologies has also reached to medicines being sold through e-pharmacies. "E-pharmacy" has not been defined in any current legislation in India. However, the Ministry of Health and Family Welfare vide a notification on 28th August, 2018 came out with a draft to amend the Drugs and Cosmetics Rules, 1945 to take into account e-pharmacies as well.
Rule 67-I of the Draft Rules defines "e-pharmacy" as "the business of distribution or sale, stock exhibit or offer for sale of drugs through the web portal or any other electronic mode." It also defines "e-pharmacy portal" as "a web or electronic portal or any other electronic mode established and maintained by the e-pharmacy registration holder to conduct the business of e-pharmacy."3 These Draft Rules not only define what type of business e-pharmacies are in but also throw light on the several ways in which the business could operate.
There are primarily two different models through which it is envisaged that an e-pharmacy would operate – Inventory-based model and the Market Place-based model.
The Inventory-based model of e-pharmacies is where the inventory of drugs and medication and service facilitators is owned by the e-pharmacy company, and the products are sold directly to the customers.
The Market Place-based model is where the tech-based company simply acts a facilitator between the buyers and sellers of medicines and drugs. The e-pharmacy acts as a platform on which authorized sellers can post their product and the seller can choose. The e-pharmacy company does not own any inventory or product and simply acts as an intermediary.
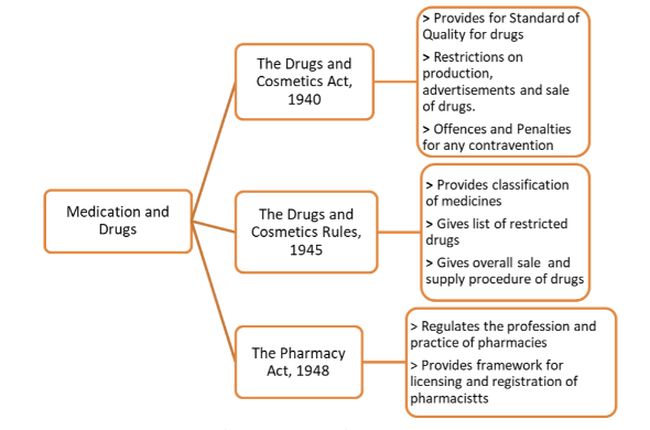
In general, an e-commerce marketplace, where all products and services are sold, are prohibited by law from advertising or selling prescribed drugs or any drugs which are in violation of the Drugs and Cosmetics Act, 1940 as they are not registered pharmacists and cannot sell certain drugs without a prescription.
No matter which model the e-pharmacy is following, a common hurdle would be that e-pharmacies would need a clear and authorized way to review prescriptions of patients to be able to sell them prescription drugs. For this, there are two major operating components that would aid them.
Firstly, there is the technology platform on which e-pharmacy would ordinarily operate. This would include the web-based or mobile-based application where the products and their price is displayed and the patient would be able to place their order and share any relevant documents if a prescription is required for a particular drug. This e-pharmacy technology will have to be approved and registered with the Central Drugs Standard Control Organization (CDSCO) and every order for a prescribed medicine has to be verified and checked by a team of registered pharmacists. Following this the registered pharmacist needs to forward the validated prescription to the pharmacy store or warehouse from where the medicines are dispensed. This web or mobile based platform will be subject to the provisions of the Information Technology Act, 2000.
Secondly, is the Pharmacy Retail Store or warehouse itself from where the medicines are dispensed. Here, the licensed store pharmacist is to check the validity of the prescription, failing which the medicines will not be dispensed. The medicines would have to be dispensed from a licensed premise in a sealed, tamper-proof pack to the patient. Along with the package a proper bill or invoice with the details of the medicines (like batch, expiry date, storage instructions, etc.) has to be provided. Overall the pharmacy retail store and warehouse will have to be operated under the oversight of the Drugs and Cosmetics Act, 1940 and Rules, 1945 and must comply with all the requirements.4
This sector being new age and largely regulated along with traditional pharmacy business, will be subject to multiple changes in law.
Current Laws and Regulations on E-Pharmacy
E-pharmacy being a new concept and business model has proven to be difficult to capture under any existing legislation or regulation.
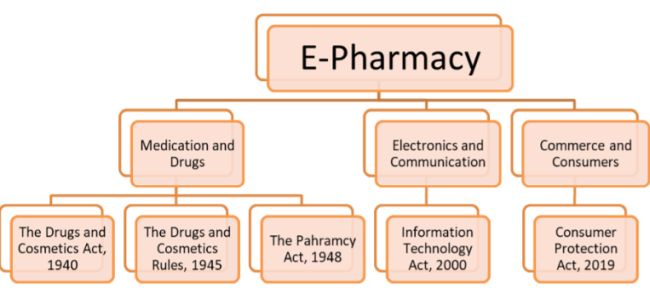
E-pharmacy: Legal Landscape
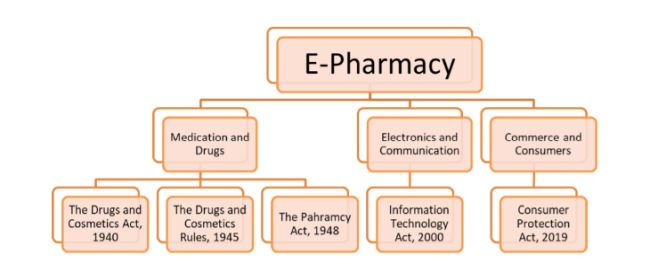
The current and primary legislation dealing with the advertising and sale of medicines is The Drugs and Cosmetics Act5, The Drugs and Cosmetics Rules6 and The Pharmacy Act.7
Pharma Legal Overview
The core legislation that deals with online matters is the Information Technology Act, 2000 ("IT Act"). It is the primary legislation through which the digital aspects of e-pharmacies will be regulated and controlled. The IT Act imposes conditions and rules which govern the way in which entities should conduct themselves when it comes to any electronic device, computer system or internet network.8 Through the IT Act and the Ministry of Electronics and Information Technology, the technical aspect of e-pharmacies can be taken care of.
When it comes to any sort of commerce, the consumers and customers are of utmost importance. Customers are important not only to the company or goods and/or service providers, but also important to the country's economy as a whole. Hence it becomes the government's duty to take charge of consumer rights. The Consumer Protection Act, 2019 is the primarily legislation on this matter and is to provide for the protection of the interests of the consumers and to administer and settle any disputes regarding consumer rights being infringed or violated.9 The Department of Consumer Affairs takes care of consumer protection issues.
Draft Rules for E-pharmacies
Drugs and Cosmetics (Amendment) Rules, 2018 (Draft Rules) which is still pending approval. Few of the objectives of these Draft Rules are:
- Require any person selling medicines through a web portal or electronic method is compulsorily required to seek a registration from the Central Licensing Authority.
- To protect the interests of the customers, require the e-pharmacy to publish information about itself like details of the owners, details of the registered pharmacist, logistics service providers and so on.
- Prevent the illegal distribution of medication online and regulate the online pharmaceutical market.
The intention to allow e-pharmacies as a legitimate business model exists and only the correct implementation procedure is being awaited from the government's side.
There have been other mentions of e-pharmacies in notifications and press releases as well. For example, the Advisory posted by the Central Consumer Protection Authority (CCPA) on 14th July, 2022 states that purchase of Ayurvedic, Siddha or Unani drugs containing ingredients listed in Schedule E(1) of the Drugs and Cosmetics Rules, 1945 are to be taken under medical supervision.10 However, it was found that such drugs were being sold to customers on e-pharmacies without any mechanism to check if it was taken under medical supervision. Hence the e-pharmacies were instructed that they would only sell such medication to those customers who upload the required prescription on their platform.
Self-Regulation
The Federation of Indian Chambers of Commerce and Industry (FICCI) had launched the Self-Regulation Code of Conduct for the E-pharmacy sector in 2016.11 These regulations are for the protection of the customers and make certain conditions mandatory; for example, the e-pharmacy can dispense prescription drugs only after the customer has uploaded their prescription on their platform and has been verified by a qualified pharmacist. It also deals with other matters like the tracing of medicines, addressing the problem of counterfeit drugs and so on. At present, these are the orders and regulations that are holding the current e-pharmacy market together.
Judicial Opinion of E-Pharmacies
Courts are looking to matters concerning and are providing orders, advisories and circulars in relation to 'online sale of drugs'. One example of this is the matter of Dr. Zaheer Ahmed v. Union of India & Others12 where the Delhi High Court gave an order and issued an advisory regarding regulation of online sale of drugs. It was held that the online sale of medicines without a license is prohibited and that it should be ensured by the All Chemist Associations of Delhi that the same is enforced.
A similar attempt to bring about regulation of e-pharmacies was made by the Madras High Court in the matter of Tamil Nadu Chemists and Druggists Association v. Union of India13. In this case, even the Hon'ble Court takes into account the advantages of e-pharmacies and why customers would prefer it. At the same time, the Court takes cognizance of the risks that are involved with the online sale of medicines – primarily the risk of selling certain drugs without a valid prescription which could have adverse side effects on the patient. Another risk the court took cognizance of, was the sale of counterfeit and compromised drugs, which could be especially detrimental to people whose daily lives depend on it.
The Hon'ble Court recognized that regulating the sale of medications online is a global problem. The Court was well aware of the proposed Drugs and Cosmetics (Amendment) Rules, 2018 and directed the Respondents to notify the Rules in the Gazette, no later than 31st January, 2019. Thereafter, the persons doing trade in online pharmacy have to obtain their licences in the manner prescribed in the rules to be notified, within a period of two months from the date of such notification.
The Courts in the above matters are able to recognize the potential and inevitability of e-pharmacies, but at the same time want to err on the side of caution as there is no preliminary legislation speaking on the matter. It is also understandable for courts to give such conservative judgements on e-pharmacies as the duty of the Judiciary is solely interpretational and not to indirectly make laws on behalf of the Legislature as that would be a breach of the separation of powers.
Global Comparison
The United States is the largest pharmaceutical market in the world, and in 2021 generated revenue of more than 560 Billion USD14. United States is also the country with some of the top global pharmaceutical companies such as Pfizer, Jhonson & Jhonson, and Merck.15 The United States is also the country with the most expensive prescription drugs.
The Federal Food, Drugs and Cosmetics Act, 1906 (FD&C Act) and Controlled Substances Act, 1971 (CSA) are the two primary legislation in the US dealing with pharmaceuticals. The former is concerned with generic medications and drugs whereas the latter is more specific towards prescriptive drugs. Being the largest pharmaceutical market in the world, and with the increase in the number of Americans using the internet, it was inevitable that medications were being sold online. The Ryan Haight Online Pharmacy Consumer Protection Act, 2008 (Ryan Haight Act) was enacted to regulate the sale and distribution of 'controlled substances' by e-pharmacies. The enactment was brought about after a teenager, Ryan Haight, overdosed on Vicodin (a prescription drug) that he had ordered from an online pharmacist and had been delivered to his house.
The Ryan Haight Act simply amended the CSA and hence does not give an extensive regulation for e-pharmacies. It does however specify that no e-pharmacy is to dispense any 'controlled substance' which is a prescription drug without the patient uploading a valid prescription. It also provides for the procedure in which the prescription will deemed to be valid; and that a signed prescription by a medical practitioner is not enough to determine its validity, and the prescription will be permissible only after an in-person examination of the patient. Thus, the law in US has specific provisions regarding the sale of prescription drugs on e-pharmacies and even has more stringent regulations than India.
In the UK, all the pharmacies, including the e-pharmacies, have to be registered with the General Pharmaceutical Council (GPhC) and need to follow the standards set out by the GPhC.16 The GPhC requires all the online pharmacies to have system in place to have an identity check for the appropriate medicine being supplied. The GPhC does not provide for a technical process to check someone's identity but rather expects the e-pharmacy itself to have an efficient and robust system in place.
For prescription-only medicines, an online pharmacy must receive a legally valid prescription before dispensing the medicine. Hence the person either needs a paper prescription or an electronic prescription via the Electronic Prescription Service (EPS) from a General Practitioner or another healthcare professional.17
Overall, e-pharmacies are widely accepted in the UK, however there is a great deal of responsibility and discretionary power placed in the hands of the e-pharmacies themselves. Though the same standards of functioning are placed on e-pharmacies as they are on any other registered brick and mortar pharmacy, the possibilities of error and abuse are higher.
Conclusion
The concept of selling medication online has been fiddled with by business-owners and customers alike for over a decade now. The government, till now, had banned the advertisement and sale of medication online by general e-commerce websites with the simple objective of protecting the customers and preventing abuse of medications.
The topography of demand and supply of medications and other commodities has changed drastically since the COVID-19 pandemic. All physical stores, including pharmacies, were virtually crippled and did not have a means to operate and get medications effectively to the patients. Though special sanctions were rolled out in favour of pharmacies during the emergency, there was still loss of lives faced by people, the government and businesses.
Greater weight being placed on online platforms for buying necessary commodities pushes even the government to legislate on such matters. Current legislation on pharmacies is not enough to deal with the complex nature of e-pharmacies simply because there are so many other sectors that come into play. The only respite that legislators do have is that they would not have to start from scratch and can take ample inspiration from already existing pharmaceutical and commercial laws.
–Nitin Jain (Partner) (assisted by Aryan Sarkar, Intern)
Footnotes
1. Drivers of Indian Pharmaceutical Exports, RBI Bulletin July 2021. (chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://rbidocs.rbi.org.in/rdocs/Bulletin/PDFs/03AR_15072021B75D322EF39B4B3A83C9B918B459A759.PDF)
2. Department of Pharmaceuticals Annual Report 2020-21.( chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://pharmaceuticals.gov.in/sites/default/files/english%20Annual%20Report%202020-21.pdf)
3. Drugs and Cosmetics (Amendment) Rules, 2018
4. E-Pharmacy in India: Last Mile Access to Medicines, FICCI. (chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://ficci.in/spdocument/20746/E-Pharmacy-in-India-Last-Mile-Access-to-Medicines_v5.pdf)
5. The Drugs and Cosmetics Act, 1940.
6. The Drugs and Cosmetics Rules, 1945.
7. The Pharmacy Act, 1948.
8. Information Technology Act, 2000.
9. The Consumer Protection Act, 2019.
10. CCPA Advisory, F. No. J-25/64/2022-CCPA.(https://pib.gov.in/PressReleasePage.aspx?PRID=1841493)
11. Voluntary Code of Conduct for E-pharmacies in India, 2016.( chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://ficci.in/pressrelease/2600/ficci-press-nov21-e-pharmacy.pdf)
12. Writ Petition No. 11711/2018
13. Writ Petition No. 28716/2018
14. U.S. Pharmacy Market Size, Share & COVID-19 Impact Analysis, Fortune Business Insights (https://www.fortunebusinessinsights.com/u-s-pharmacy-market-106306).
15. Global pharmaceutical sales from 2017 to 2021. (https://www.statista.com/statistics/272181/world-pharmaceutical-sales-by-region/)
16. Standards for Registered Pharmacies (revised June 2018).
17. Dangers of buying medicines online, NHS. (https://www.nhs.uk/nhs-services/prescriptions-and-pharmacies/pharmacies/dangers-of-buying-medicines-online/)
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


