Battery technology will play a critical role in the future of the global energy markets, in everything from electric vehicles to grid-scale batteries. Many countries, including the US, have set ambitious climate goals which can only be achieved through the use of diverse energy generation and storage mechanisms. For example, the Biden-Harris administration has set a goal that 50% of all new vehicles sold will be electric by the year 2030. U.S. Government Agencies have actively supported development in this area, including the U.S. Patent and Trademark Office's expanded and extended Climate Change Mitigation Pilot Program which aims to expedite examination of patent applications directed to climate technologies, and the numerous avenues of support provided by the U.S. Department of Energy (DOE), such as direct grant funding for battery technology (e.g., here and here), energy storage and battery research centers at Argonne, Oak Ridge, Lawrence Berkeley, and Pacific Northwest National Labs, and through funding from the Advanced Research Projects Agency – Energy (ARPA-E).
Achieving climate goals will require significant capital investment into the development and scaling of improved battery technologies. The Inflation Reduction Act (IRA), enacted in August 2022, allocated billions of dollars for EV-friendly infrastructure and manufacturing to be distributed through agencies including the U.S. Departments of Transportation and Energy. In addition to (and as a result of) this capital investment, significant technological advances are still needed in several key battery technology areas while existing core or "legacy" technologies proliferate to mainstream use. While battery technology has advanced rapidly in the past decades, further advances are likely necessary to complete the energy transition.
Background
The majority of legacy battery technology relies on lithium-ion chemistry originally developed in the 1960s, and for which John B. Goodenough, M. Stanley Whittingham and Akira Yoshino were awarded the 2019 Nobel Prize in Chemistry. The fundamental chemistry at the core of the early technology is still used in the vast majority of batteries today.
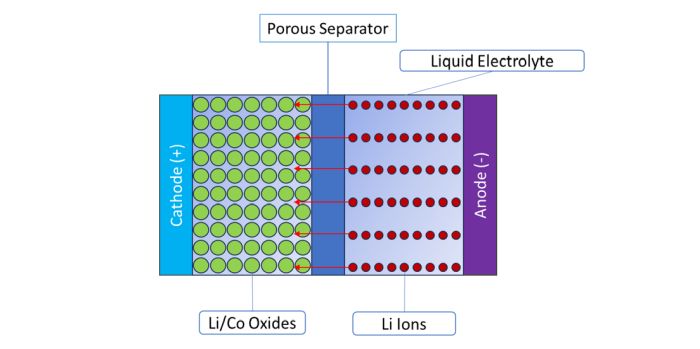
Each cell of a lithium-ion battery contains a cathode, which is the positive terminal of the battery and is typically made from lithium/metal oxides (usually lithium/cobalt); and an anode, which is the negative terminal of the battery, and is traditionally made from graphite or a graphite/silicon composite. Inside these terminals, on the cathode side, is a matrix of lithium/metal oxides (usually lithium/cobalt). On the anode side, positively charged lithium ions are suspended in a second matrix, usually made up of lithium and carbon. Both sides are filled with a liquid electrolyte, and the two sides of the battery are separated by a porous separator that allows the lithium ions to move from one side of the battery to the other. When the battery is discharging (putting out power), positively charged lithium ions move from the anode side to the cathode side, allowing negatively charged electrons to leave the anode and power the load. The battery is fully discharged when all of the lithium ions have moved to the cathode side of the battery. When the battery is charging (taking in power), the positively charged lithium ions move from the cathode side to the anode side, while negatively charged electrons flow into the battery through the anode.
Legacy Battery Technology
Older styles of lithium-ion batteries have limited applications due to size and material properties. Scaling lithium-ion batteries to power larger devices requires the use of multiple (often thousands in the case of an EV) battery cells connected in series. Given the limitations on how small these cells can be manufactured, the number of cells required to power large vehicles is often prohibitive, resulting in batteries that are too large or heavy to be practical. For example, experts estimate that batteries based on lithium ion technology would need to reach much higher energy densities – around five-times their current best – to be able to power small aircraft. Further, the flammable metals and liquid electrolytes used in lithium-ion batteries can make them less desirable for applications where they would need to operate under extreme heat. There are also concerns with sourcing and importation of raw materials for older styles of lithium ion batteries, which can result in increased costs and supply chain delays.
When improving and developing new battery technologies, developers will likely focus on domestically-sourced materials (to align with the Federal Government's stated goals relating to energy independence), which can help make batteries that are cost-competitive with legacy technologies like internal combustion engines in mobile vehicles or natural gas or coal for grid applications. Further, larger capacity batteries are a critical part of widescale adoption of renewables, as many of these technologies (e.g., wind, solar) employ a peak/valley style of production, which requires energy storage to store excess energy produced during peak times (windy periods or daylight) to compensate for downtimes in production (periods without wind or night time). As is generally the case, developments in the technology (in this case, batteries) have been guided by the known requirements of the end-use, e.g., size, weight, energy density, and cost for EV applications, and capacity, longevity, and durability for electric grid applications. Thus, there is no one-size-fits-all battery, and advancements have been made in a number of unique core areas targeting each application where advanced batteries are urgently needed.
Developments in Battery Technology
Given the level of financial incentives and structural support now available in this area, a number of companies have started to focus on the development of new technologies needed to scale renewables. While there are many new areas of advancement in battery technology, we have chosen to focus on the following areas of development, which are among the most promising.
Improved Lithium-Ion Batteries
In recent years, a number of key advancements have been made to lithium-ion batteries which improve their energy density, charging speed, and lifespan. Particularly, the integration of nanoparticles into the anodes of lithium-ion batteries (particularly silicon nanoparticles) has significantly improved capacity, stability, and cycling performance. These materials allow batteries to store higher concentrations of lithium ions with less frequent charging, potentially making them more suitable for both grid-scale and EV applications.
Additionally, the nickel manganese cobalt (NMC) cathode materials used in older styles of lithium-ion batteries can be expensive and challenging to source. Development of cheaper lithium iron phosphate (LFP) cathode materials substantially lower cost and streamline the supply chain for lithium-ion batteries, leading to wide adoption in the EV market. Overall, dlthium-ion batteries are also the cheapest and most market-ready technology given their long history of use, and their safety thresholds are well understood.
Solid-State Batteries
Solid-State (SS) batteries have similar architecture to lithium-ion batteries, but use a solid separator such as ceramic or glass between solid electrodes, rather than the liquid electrolyte used in legacy batteries. Numerous versions of these SS batteries are currently in development, including batteries with cathodes made from nickel-rich materials, and anodes made from graphite, silicon, or lithium metal. Though there are certainly a number of developmental obstacles to surmount before commercial deployment of SS batteries, the fundamental technology holds several promising advantages over legacy batteries.
The SS battery architecture provides significant advantages over legacy lithium-ion batteries, specifically through the variation in anode/cathode materials. As discussed above, the use of cobalt in the cathode of legacy batteries raises sourcing concerns, so batteries that can entirely circumvent the need for cobalt could eliminate these issues. Further, the use of lithium metal anodes (rather than graphite/silicon) has long been a goal of the battery industry due to their potential to enable significantly improved performance, safety, recyclability, and lower cost. These types of batteries would be highly promising, particularly for mobile applications, if their development continues on its current trajectory.
Sodium-Ion Batteries
Sodium-ion batteries are similar to traditional lithium-ion batteries, but with sodium ions being used in the place of traditional lithium ions. This enables the use of new cathode materials made from sodium nickel-manganese-iron (NMF) oxides. Sodium is far more abundant and easier to mine than lithium, so overall the materials for these batteries will be easier to source, cheaper, and less susceptible to price fluctuations and supply chain disruptions. However, these new cathode materials still use cobalt. Additionally, sodium is significantly more dense than lithium, so these batteries are roughly three times heavier than their lithium counterparts, making them less suitable for mobile applications like EVs; they could, however, still be suitable for stationary use in home batteries or grid-scale applications, where weight and size are less important than cost and long-term stability.
Iron-Based Batteries
Iron-based batteries, and particularly iron flow and iron-air batteries, operate on a similar principle to lithium-ion batteries, but use the flow of iron electrolyte solutions across a membrane, or the "reversible rusting" of iron with oxygen, to generate electric current instead of legacy lithium chemistries. The difference in the chemistry of the cell results in several distinct differences in the iron-flow cells over their lithium counterparts – namely, wider operating temperature ranges, longer lifetimes, decreased safety risk, higher storage capacity, and cheaper component elements.
This technology offers some significant advantages, particularly given the existing iron supply chain within the US, resulting in extremely secure domestic sourcing. And while Iron-based batteries are much larger in size and significantly heavier than their lithium counterparts, their larger capacities make them well-suited to grid use, where battery "footprint" is less important than in mobile vehicle applications. Iron-flow batteries store around twelve hours of electricity and iron-air up to around 100 hours at a home- or grid-scale output level. In contrast, lithium-ion batteries currently used in electric utility applications typically store enough power for just a few hours at a time. This shorter duration capability is workable for their current role in supplementing short-lived frequency fluctuations, but would not be a sustainable solution for an entirely alternative energy grid, which would require much larger battery storage capacities that would allow for longer periods of battery discharge while renewable production is low (e.g. when periods of high demand coincide with low winds or short winter days).
Many experts agree that the chemistry of iron-flow/iron-air batteries makes them ideal candidates for supporting an alternative energy-fueled grid, compounded by their safety and longevity advantages that would further benefit this type of application. Therefore, this class of batteries appears destined to support in home use and grid-based applications.
Summary
Battery technologies are poised to make significant advances in the next few years fueled by an exciting combination of emerging government policy, market forces, and scientific discoveries. We expect that, as with most technologies involved with the transition to renewables, multiple different types of technologies, coupled with technological advances on multiple fronts, will be necessary to account for needs across different applications such as EVs, home batteries, and grid-scale applications. In batteries for EV applications, advances will continue making batteries smaller, lighter, cheaper, and more capacious, while for home- and grid-use applications, the focus will be on longer lifetimes, higher safety/stability, and charge/discharge profiles better suited to the demands of the use. It is truly an exciting time to keep an eye on the energy storage industry.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




