CRISPR is a gene editing technique that promises to revolutionize genetic engineering, but already is raising ethical, business, and legal issues. This is the first in a monthly series of articles on CRISPR. If you are interested in more information, please download a digital copy of our web conference on "Navigating the CRISPR Patent Landscape and Business Impact" or contact any of the authors.
About CRISPR
The acronym CRISPR stands for clustered regularly interspaced short palindromic repeats, which refers to a pattern of DNA sequences observed in a wide range of bacteria and archaea, where a repeated sequence is interspersed with shorter sequences that correspond to viral DNA sequences. The CRISPR pattern is believed to be associated with a prokaryotic immune defense mechanism that helps the bacteria (or archaea) recognize and attack viral DNA. As part of this immune defense mechanism, these prokaryotes also have genes that encode CRISPR-associated proteins (Cas), that precisely cut target viral DNA sequences and prevent invading viruses from replicating.
The CRISPR system is being applied to genetic engineering by using a Cas protein, such as the Cas9 nuclease from Streptococcus pyogenes, in association with one or more guide RNAs which tell Cas9 precisely where to snip a target DNA sequence. As discussed in Cong et al., Science 339:819-823 (2013), and Mali et al., Science 339:823-826 (2013), this methodology can be used in eukaryotic cells to edit a eukaryotic genome, including a human genome. Because of the excellent editing efficiency and ease of use, CRISPR has been widely hailed as one of the most important biotech discoveries of the 21st century.
CRISPR Clinical Trials
Many groups around the world are actively seeking to harness the power of CRISPR for therapeutic applications. It has been reported that the first human CRISPR clinical trial will soon start in China, in which the CRISPR system will be used to edit T cells of lung cancer patients ex vivo. It also has been reported that a human CRISPR clinical trial has received approval to proceed in the U.S.
U.S. CRISPR Patents and Published Patent Applications in the U.S.
We searched the USPTO database for U.S. patents and published applications that recite "CRISPR" or "Cas9" in either the claims or the abstract. Our search identified 35 CRISPR-related granted U.S. patents and 305 CRISPR-related U.S. patent applications that had been published as of July 27, 2016. (The search results are available here.) This figure highlights the exponential increase in CRISPR-related U.S. patent filings.
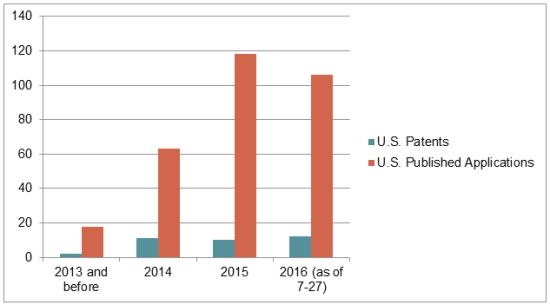
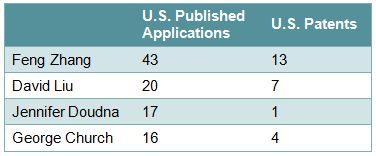
Applicants/Assignees who appear to be actively filing in this area include, on the university side, Harvard, MIT, the Broad Institute, and the University of California (Table 1), and on the corporate side, Sangamo, Caribou, Regeneron, Dow, and Agilent (Table 2). Some of the inventors include Feng Zhang of the Broad Institute, David Liu and George Church of Harvard, and Jennifer Doudna of the University of California (Table 3).
Table 1 – Selected University Applicants
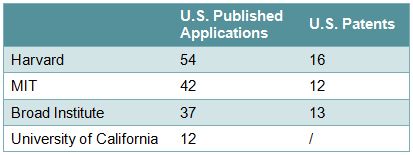
Table 2 – Selected Corporate Applicants
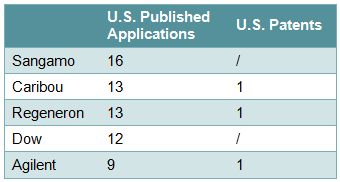
Table 3 – Selected Inventors
CRISPR Legislative News
Earlier in July 2106, the Senate passed legislation (S.764) that would require the Secretary of Agriculture to establish a national standard for disclosing "bioengineered" foods that could have implications for CRISPR.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



