India has a thriving pharmaceutical industry, which is grown in leaps and bounds in the last few years. Its importance to the world as a whole cannot be overstated - it is for good reason many people refer to India as the World's Pharmacy or consider it to be the world's greatest vaccine producer. The latter has become very evident in the last few months, as the rate of production of COVID-19 vaccines in India has surpassed all global expectations. Leading private sector companies like the Serum Institute and Bharat Biotech are now on the tips of everyone's tongues globally. It was in fact recently reported by the Indian Brand Equity Foundation (IBEF) that the Indian pharmaceutical sector supplies over 50% of the global demand for vaccines. It has also been forecasted that the Indian pharmaceutical sector would reach a staggering value of around 120-130 billion USD by 20301. While the sector is indeed booming and is burgeoning with possibilities of reaching greater heights than ever. With the increased importance and global role of the Indian pharmaceutical sector, attention would inevitably be directed to the status of intellectual property, especially trademark law and general branding practices, in India. In this regard, a dispute regarding two similar-sounding pharmaceutical names/trademarks, i.e. Dispovan v. Dipcovan was reported in the Indian media. This piece will analyze the said dispute, especially with the eyes of the earlier important judicial precedents regarding the subject matter.
Pharmaceutical Trademark Similarity
DISPOVAN V. DIPCOVAN
It has been recently widely reported in many leading Indian Dailies that there was a trademark dispute between the government-run organization Defence Research and Development Organisation (DRDO)/ Vanguard Diagnostics and Hindustan Syringes and Medical Devices (HMD), which is the maker of the quite famous syringe brand DISPOVAN.
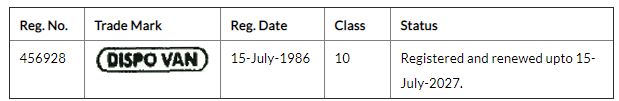
Dispovan is quite a famous brand of single-use disposable syringes, which many readers may in fact have encountered during vaccination and other injection-based medical procedures. HMD was in fact reportedly in receipt of an order from the Indian Government for the supply of 265 million auto disposable syringes2. HMD is also the owner of the below trademark registration for the DISPOVAN mark in India:
Whereas, DRDO is India's premier government agency for a wide variety of research and development, including but not limited to for advanced weaponry such as missiles to medical components. It has in fact been actively aiding in the fight against COVID-19 and has reportedly recently released an Anti-Covid drug3.
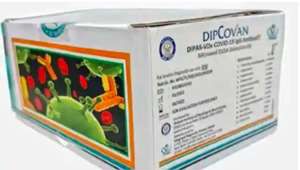
DRDO, in collaboration with the indigenous diagnostic company Vanguard Diagnostics, had recently developed and launched a COVID antibody detection kit, under the brand name DIPCOVAN, purportedly with the below packaging4:
Vanguard Diagnostics had also filed the below application for the aforesaid brand name:
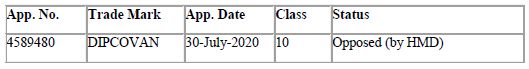
Due to the prima facie similarity between the marks DISPOVAN and DIPCOVAN, and considering that they both fall under class 10 of the Nice Classification, it seems inevitable that a trademark dispute would arise regarding the same, which it reportedly did. The aforesaid application for DIPCOVAN was indeed opposed by HMD (vide opposition no. 1075355 on November 26, 2020). Further, reports on various news outlets suggest that HMD may have also approached DRDO/ Vanguard to urge them to adopt a different name for their product, likely by sending a Cease & Desist Letter5.
MATTER RESOLVED
It has now been reported that the matter between HMD and DRDO/ Vanguard has been amicably resolved, leading to the resultant withdrawal of the trademark application no. 4589480 for the mark DIPCOVAN, vide a letter of withdrawal which can be accessed from the Trade Marks Registry's website at https://ipindiaonline.gov.in/eregister/showdocument.aspx?DOCUMENT_NO=WFlaW1xdKS4qLCorMClYWVpbXF0=.
TRADEMARK INFRINGEMENT/ PASSING-OFF IN THE PHARMACEUTICAL SECTOR - TRENDS
There have been a plethora of cases over the years in India, which have dealt with trademark infringement and passing-off in the field of pharmaceuticals. One commonality in this ample body of jurisprudence is that the benchmark of the likelihood of confusion in cases involving pharmaceuticals has to be minimum or even bare minimum. This principle was elaborated in great detail by the Supreme Court of India in the landmark case of Cadila Health care Ltd. v. Cadila Pharmaceuticals Ltd.6 The fact that the threshold of the likelihood of confusion is quite low for such marks and cases can also be seen from the recent case of Mankind Pharma Limited v. Novakind BioSciences Private Limited, wherein the Hon'ble High Court of Delhi had granted an ex-parte ad-interim injunction in favor of the Plaintiff (Mankind), restraining the defendant from, inter alia, manufacturing and selling any pharmaceutical product bearing the suffice 'KIND'. In this case, the Plaintiff was the proprietor of the well-known trademark MANKIND and various other marks with the suffix KIND, whereas the defendant was using the mark DEFZAKIND for its pharmaceutical preparation/medicine. For more information on this case, please visit our earlier article here.
Thus, in the present case of DISPOVAN V. DIPCOVAN, it is quite likely that had the matter gone to Court, the Court would have ruled in favor of HMD.
Footnotes
1. Indian Pharmaceuticals Industry Analysis: https://www.ibef.org/industry/indian-pharmaceuticals-industry-analysis-presentation#:~:text=India's%20drugs%20and%20pharmaceuticals%20exports,US%24%2025%20billion%20by%202025.
2. Covid-19 vaccination: HMD bags govt order for supply of 265 million auto disposable syringes, Business Today: https://www.businesstoday.in/coronavirus/covid-19-vaccination-hmd-bags-govt-order-265-million-auto-disposable-syringe/story/433558.html
3. DRDO's anti-Covid drug 2-DG: Second batch of 10,000 packets to be released tomorrow, Live Mint: https://www.livemint.com/news/india/drdos-anti-covid-drug-2-dg-second-batch-of-10-000-packets-to-be-released-tomorrow-11622037866917.html
4. DRDO unit develops antibody detection-based kit 'Dipcovan', Live Mint: https://www.livemint.com/news/india/drdo-unit-develops-antibody-detection-based-kit-dipcovan-11621605550195.html
5. Dispovan owner to urge DRDO for new name of Covid anti-body kit: Punjab News Express - https://www.punjabnewsexpress.com/health/news/dispovan-owner-to-urge-drdo-for-new-name-of-covid-anti-body-kit-138375 ; 'DRDO Dipcovan brand too similar to Dispovan' - Syringe maker warns govt agency of legal action, ThePrint.in - https://theprint.in/health/drdo-dipcovan-brand-too-similar-to-dispovan-syringe-maker-warns-govt-agency-of-legal-action/663425/
6. Cadila Health care Ltd. v. Cadila Pharmaceuticals Ltd., (2001) 5 SCC 73
For further information please contact at S.S Rana & Co. email: info@ssrana.in or call at (+91- 11 4012 3000). Our website can be accessed at www.ssrana.in
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

