Ministerial Order 39-C/2024 of 2 February was published in order to define the reference countries to be taken into account in 2024 and the exceptional criteria to be applied in the review of the prices of medicines.
Reference countries for new prices and for the annual price revision
- In 2024, the reference countries for the approval of new prices and the annual price review of medicines (outpatient and hospital market) will be maintained: Spain, France, Italy and Slovenia.
Exceptional criteria to be observed in the Annual Review of Medicine Prices (APR) 2024
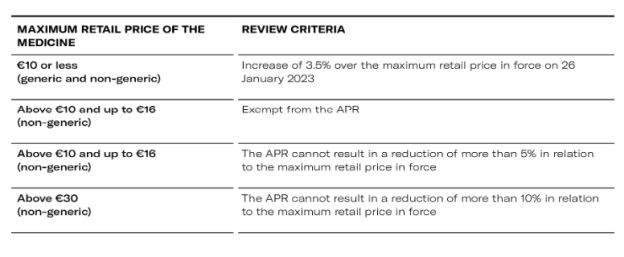
- Outpatient market
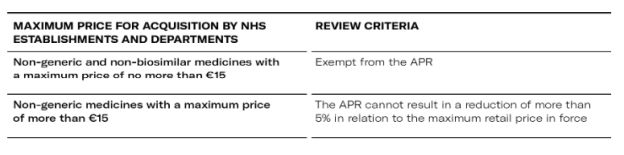
- Hospital market (non-generic and non-biosimilar)
- Outpatient1and hospital market (generic and biosimilar)
All generic and biosimilar medicines are exempt from the APR, except for generic medicines with a maximum price of €16 or more and which is higher than the maximum price of the reference medicine resulting from the 2024 APR or the 3.5% increase (if applicable). In this case, the maximum price of the generic medicine cannot exceed the maximum price of the reference medicine.
Deadlines for marketing authorisation holders or their legal representatives to submit the prices to be applied in 2024:
- Annual review of the maximum retail price of non-generic medicines: By 15 February 2024, with prices taking effect on the following 1 March
- Annual review of maximum retail price for generic medicines: By 29 February 2024, with prices taking effect on the following 1 March
Footnote
1. Generic medicines on the outpatient market, with a price of €10 or less, can increase by up to 3.5% compared to the maximum retail price in force on 2 February 2024.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




